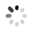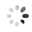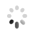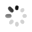CitrusGreening Research Highlights
We feature important developments in Huanglongbing (HLB) research in this section. Please contact us to submit your research.
Hunter et al. 2019
New strategies, using Modified Pest Management, MPM, approaches continue to provide highly specific pest control strategies that are safe. The method was highly specific to the psyllid with no detrimental impact on beneficial insects, predators or parasitoids of psyllids. Similar management will someday reduce dependence upon broad spectrum insecticides. The breakthrough permits treatment of adults to disrupt an essential insect protein. The method, called BAPC-assisted-Delivery, improved treatment of adult Asian Citrus Psyllids, Diaphorina citri, whose offspring then showed the same greatly reduced fecundity and lifespan. The method also permits researchers to screen other potential targets for new pest control products, and to understand a genes specific function in psyllid biology, or in the bacterial pathogen acquisition and transmission to citrus trees. Using this method a specific gene was identified that may provide one means to greatly reduce Asian citrus psyllid populations, and thereby reduce the spread of Huanglongbing.
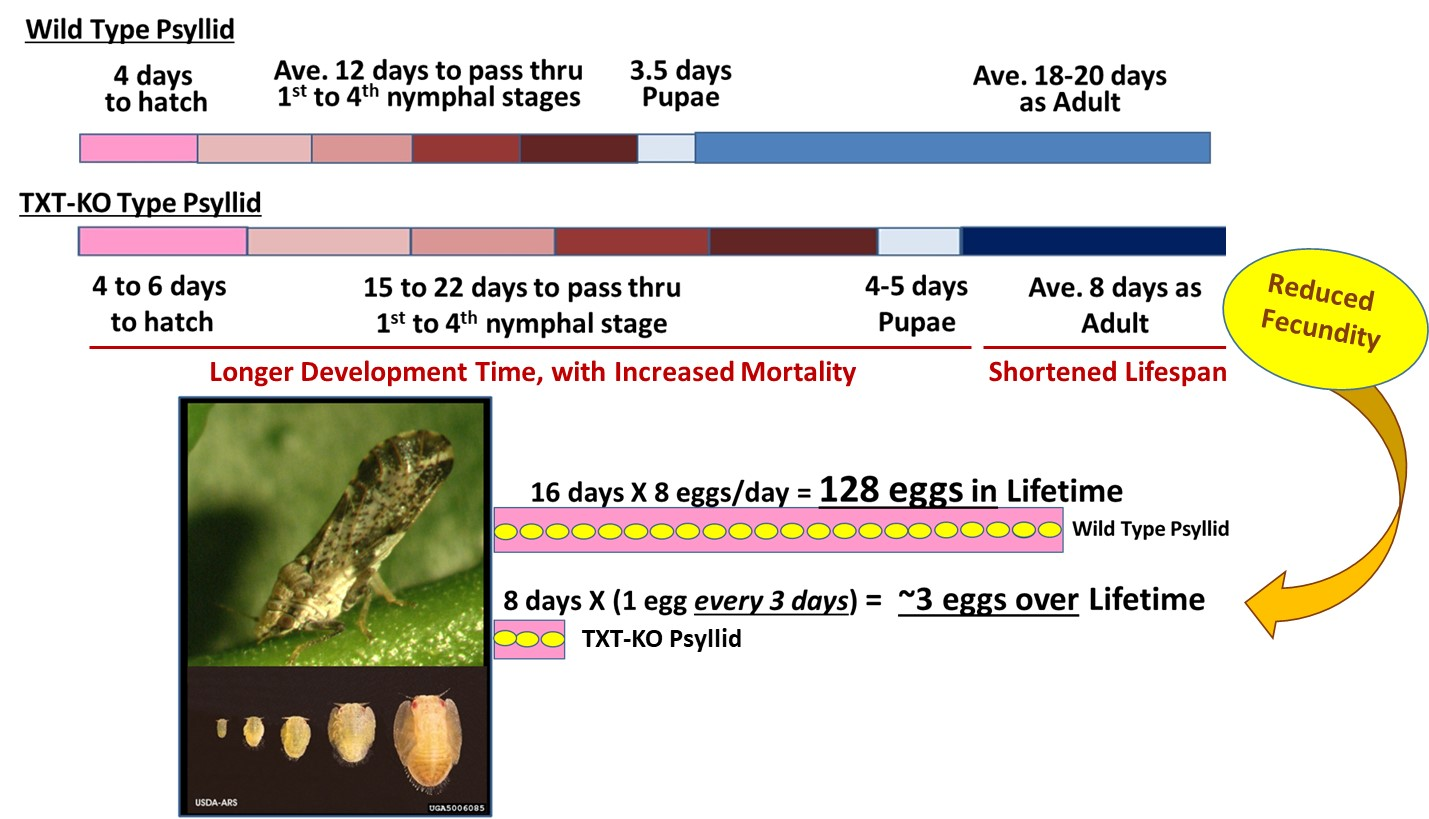
Figure 1. The G1, TXT-KO adult psyllids had significantly shorter adult lifespans post eclosion living an average 8.5 days, compared to controls injected with buffer, which averaged 16 d post eclosion. Adult female insects producing eggs when injected resulted in TXT-KO G1 mutants. The few adults G2, that eclosed from their eggs also showed the traits of longer development time, increased mortality, and shortened adult lifespan as adults, with greatly reduced fecundity, as compared to wild type psyllids.
Hunter et al. developed BAPC-assisted-CRISPR-Delivery System, for improved heritable gene editing when injecting adults or nymphs of insects [Hemiptera]. Addition of Branched Amphiphilic Peptide Capsules, BAPCtofectTM (PhoreusTM Biotechnology, Inc.) improved delivery of CRISPR components for heritable gene editing, and also plasmids, or dsRNA for expression and gene targeting in insect nymphs and Asian Citrus Psyllid adults. The method bypasses traditional microinjection of eggs. This reports the first heritable gene knock out (KO), using BAPC-assisted-CRISPR-Cas9, which resulted in G2 mutants post injection of adult psyllid females, G0. The KO target was the thioredoxin gene, resulting in nucleotide deletions at the sgRNA at each end of the 550 nt region in the gDNA. In psyllids the thioredoxin gene, TXT, knock outs produced changes in physiology. Strategies to alter the psyllid vector, into a non-vector of the bacterium, CLas, are now possible. One function of TXT is to promote development. The TXT-KO adult lifespan was reduced by ~50%, development was slower and fecundity severely limited to just a few eggs in the KO adults lifetime. Reduced fecundity and slow development traits is a strategy that would reduce psyllid populations by increasing exposure time to parasitism and predation on nymphal stages. Classical CRISPR/Cas9 injections of nymphs, pupae, and adults produced white eye phenotypes, by vermillion gene knock out (Hunter in press). The BAPCTM-assisted delivery system advances gene editing across hemipterans and insects by permitting the use of nymphs and adults. To read about these findings in more detail, please visit FASEB journal.
Mann et al. 2018

Diaphorina citri Nymphs are Resistant to Morphological Changes Induced by "Candidatus Liberibacter asiaticus" in Midgut Epithelial Cells
Translation? The gut cells of the Asian citrus psyllid nymphs do not respond the same way to infection by the Huanglongbing bacterial pathogen as adult guts. This was unexpected.
Translation? The gut cells of the Asian citrus psyllid nymphs do not respond the same way to infection by the Huanglongbing bacterial pathogen as adult guts. This was unexpected.
Marina Mann and Somayeh Fattah-Hosseini, two scientists in Michelle Heck's vector biology lab (located in Ithaca, NY at the Boyce Thompson Institute for Plant Research) lead research that found a surprising visual difference between how nymph and adult guts of the Asian citrus psyllid (Diaphorina citri) respond to infection by "Ca. Liberibacter asiaticus," the causative bacterial agent of Huanglongbing (HLB), or Citrus greening disease.
Mann et. al. used the confocal microscope to image guts that were stained with a fluorescent dye to show the location of the nucleus in each gut cell. They looked at four treatments: healthy nymphs and adults, and "Ca. Liberibacter asiaticus"-exposed nymphs and adults. By comparing images of individual guts in each treatment they could define four "fragmentation classes" of nuclear disturbance (classes 0-3), ranging from the healthy, round nuclear shape, to completely fragmented and barely recognizable nuclear shapes. (Figure 3 of paper). Then, they counted every single nucleus in each of their gut images and scored it 0-3 to get the average proportion of nuclei in each fragmentation class, for each of their treatments.
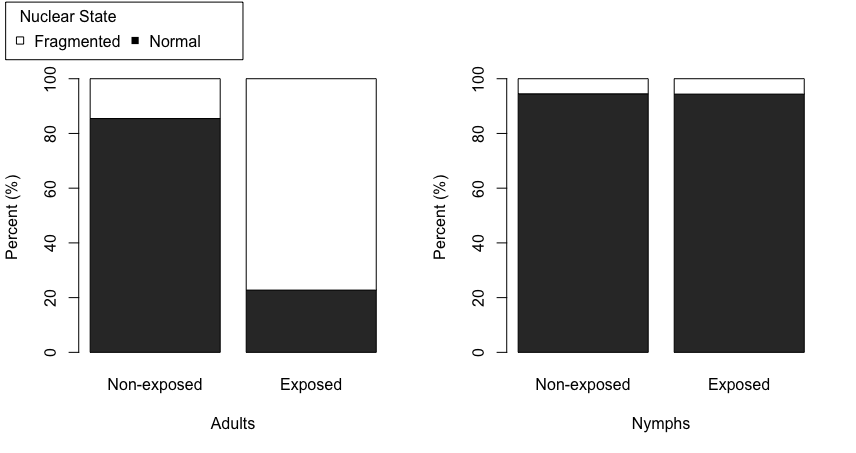
In support of results published earlier by Kruse et al (2017), adult psyllid guts, when exposed to the HLB bacteria, were shown to have a marked increase in numbers of gut nuclei that were non-normal (classes 1-3, excluding 0) when compared to adult guts from healthy psyllids. This elevated visual gut disturbance was expected. (Figure 5 of paper).
However, when they looked at guts of both healthy and "Ca. Liberibacter asiaticus"-exposed nymphs, the guts looked the same. There was no visible impact of infection on gut nuclear shape of nymphs, a striking difference to what was seen with adults. (Figure 5 of paper).
The literature solidly shows that Asian citrus psyllids acquire the HLB bacteria much better as nymphs than as adults. This result, that nymphs do not have a visible response to infection, suggests that "Ca. Liberibacter asiaticus" has found a "backdoor" to evading the psyllid immune response. Identifying this door may be a viable means of controlling the psyllid's ability to vector the bacterial disease.
To put these results into more perspective, Mann et. al. furthered their analysis by imaging the psyllid endosymbiont Wolbachia, as well as the HLB pathogen itself "Ca. Liberibacter asiaticus," in the guts of adults and nymphs (as opposed to imaging just the gut nuclei). To read more on these specific results and the author's discussion, please see the full article: Mann et al. 2018.
Kruse et al. 2017
The Asian citrus psyllid is the vector for the bacterium associated with citrus greening disease, Candidatus Liberibacter asiaticus (CLas). CLas is spread by psyllids through a process called circulative transmission. This means that as the insects feed on plant sap, CLas moves into the insect gut and crosses it to exit into the hemolymph (insect blood). It circulates in the blood and finally reaches the salivary glands to be spit back into the next host tree. Kruse and her colleagues were interested in how CLas interacts with the gut to cross into the blood and be transmitted, and if the presence of CLas causes changes in the cells of the gut.
To find out, researchers at the Boyce Thompson Institute and Cornell University began with hundreds of dissected guts from psyllids feeding on either healthy or CLas-infected citrus plants. They analyzed their transcriptome (the genes being expressed in these tissues) and proteome (the proteins present in the tissues). This gives a snapshot of the activity in gut cells when CLas is present.
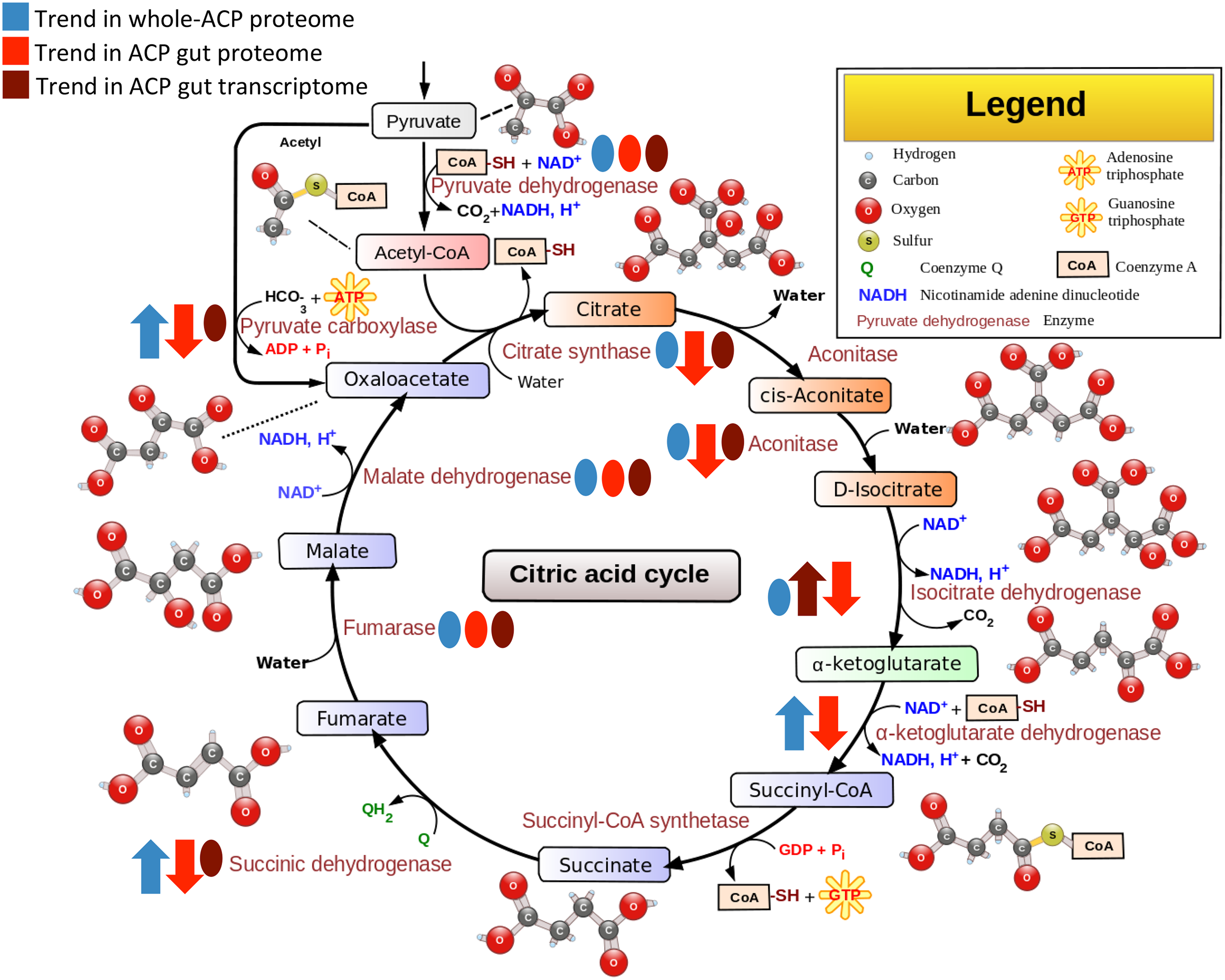
Kruse et. al. found that when CLas is in the insect gut, gut cells cannot make as much cellular energy. This became clear when they saw changes in the cycle that mitochondria use to make energy-it's called the citric acid cycle (Figure 1). Almost every protein in the cycle was effected by CLas! The gut cells also seem to react to CLas as a threat, rather than just a hitchhiker traveling from tree-to-tree. The cells mount an immune response to CLas. The cells even produce some defensive proteins that contribute to insecticide resistance.
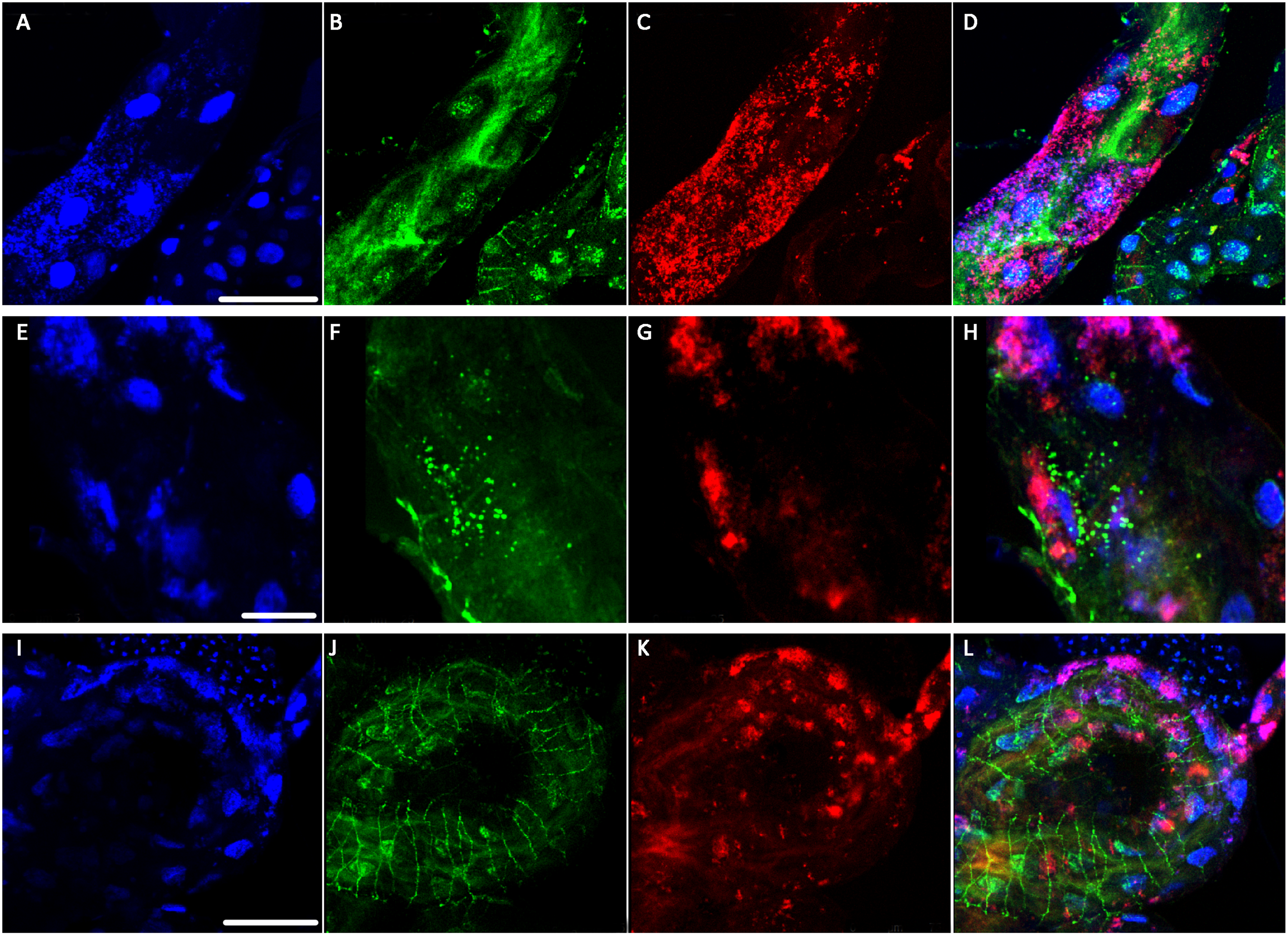
The researchers also used a confocal microscope to look at specific bacteria inside the psyllid guts. They used probes which tag bacteria with a colored fluorescent molecule. This way, they could tell different bacteria apart and see exactly where they are. They used a green probe for CLas (Figure 2B), and a red probe for a symbiotic bacteria called Wolbachia that is always in the psyllid gut (Figure 2C). The cell's nuclei were labeled in blue (Figure 2A). They found that CLas enters psyllid gut cells. They also saw that Wolbachia and CLas can be in the very same gut cell, but they never seem to overlap (Figure 2D). This suggests that Wolbachia and CLas are either competing or cooperating within the psyllid. This begs the question-could Wolbachia be a potential tool in the fight against CLas, or are Wolbachia and CLas partners in crime? To read about these findings in more detail, find the full article at Plos One.